Imbibe Explains FDA’s New “Healthy” Proposal with Essential Considerations and Potential Effects on Your Food and Beverage Innovations – Part 1
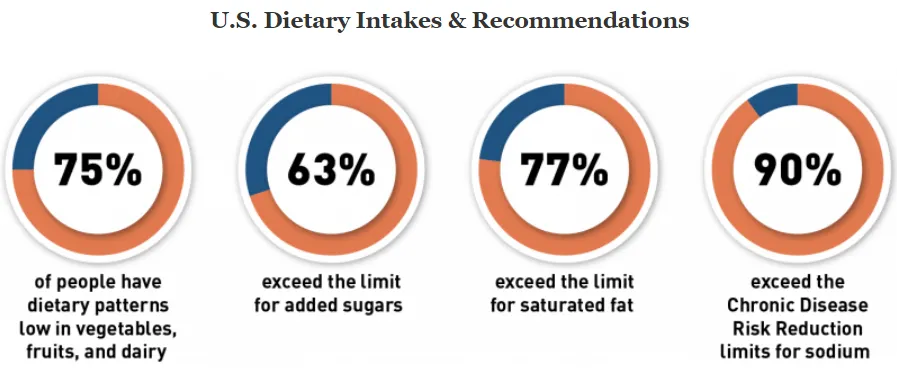
In 2022, the FDA proposed an update to the existing nutrient content claim “healthy” – first established in 1994, based on updated nutrition science. The effort is one of the agencies nutrition initiatives that seeks to foster a healthier food supply and reduce chronic diseases related to diet — the leading cause of death and disability in the U.S. The FDA states that more than 80% of people in the US aren’t consuming enough vegetables, fruits and dairy, and most Americans are consuming too much sodium, saturated fat, and sugar. By empowering consumers to make healthier choices and improve dietary patterns, they hope to advance health equity and decrease occurrences of heart disease, type 2 diabetes, certain cancers, and obesity.
How is Healthy Defined Today?
The 2020-2025 Dietary Guidelines for Americans emphasizes the “importance of a healthy dietary pattern as a whole — rather than on individual nutrients, foods, or food groups in isolation.” The existing definition focuses on maximums for saturated fat, total fat, cholesterol, and sodium, and excludes foods considered healthy today such as nuts, salmon, avocados, and water, while including high-sugar, low-fat foods as healthy. It also states that foods must contain a minimum of 10% Daily Value for at least one of the following nutrients: vitamin A, vitamin C, calcium, iron, protein, and fiber. Currently, only 5% of packaged foods are labeled as healthy, according to the FDA.
FDA: Use of the Term Healthy on Food Labeling
What is the Proposed Definition of Healthy?
Though the FDA states that the update adheres to current nutrition science, federal dietary guidelines, and the updated Nutritional Facts label, it does shift focus to nutrient-related criteria including sugars, sodium, and the type of fat rather than total amount consumed. Whole grains, lower-fat dairy, protein foods, and healthy oils like olive and canola will be considered healthy. Nutrient dense raw whole fruits and vegetables, water, and carbonated water automatically qualify. Label Insights says, “13% of food products in its 270,000-item database would now qualify as healthy up from 10.3% currently.”
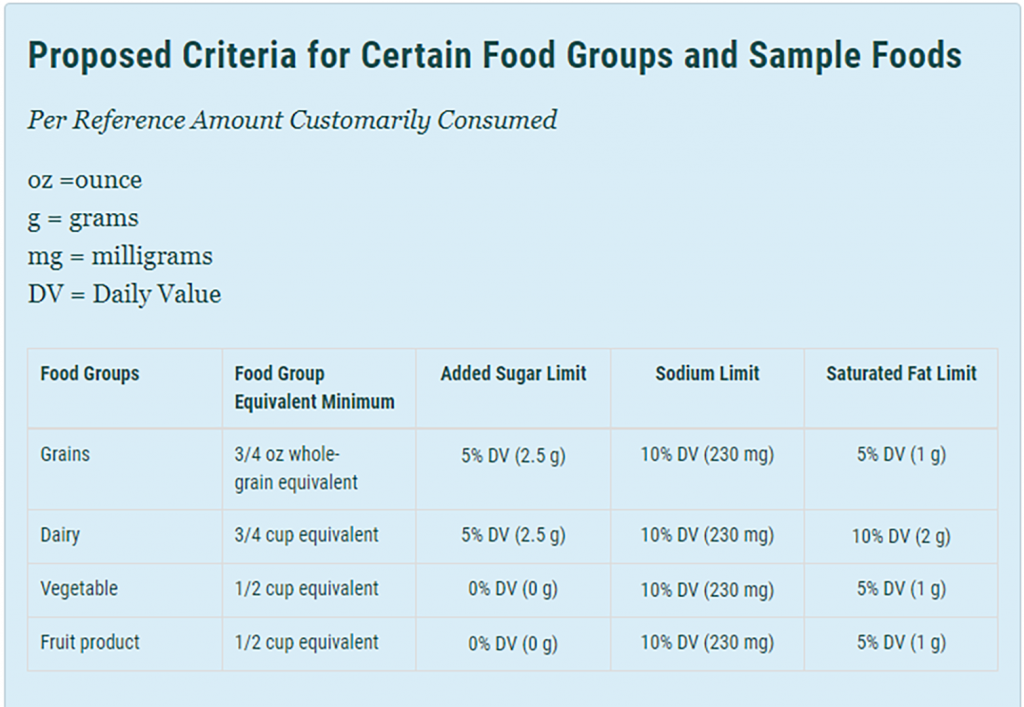
To meet the proposed healthy definition, products cannot exceed certain amounts of sugar, sodium, and saturated fats (based on a percentage of the Daily Value) and must contain a “meaningful amount” of food from at least one food group or subgroup recommended by the Dietary Guidelines. The food measurements from each group referred to as “food group equivalents,” vary for single products, mixed products (comprised of more than one food group), and main dishes and meals, which receive the greatest flexibility. They are based on a Reference Amount Customarily Consumed (RACC), which determines serving size. These limits vary based on the type of food that the product contains – e.g., vegetable-based products have a lower added sugar limit than a grain-based product. The agency has made available the Proposed Criteria for Certain Foods and Sample Foods chart and includes the example that a cereal would need to contain ¾ ounces of whole grains and contain no more than 1 gram of saturated fat, 230 milligrams of sodium and 2.5 grams of added sugars per serving. Based on the new definition, cereals like Raisin Bran, Corn Flakes, Honey Nut Cheerios, and Special K will be restricted from labeling their products healthy. These cereals contain between 4-12 grams of sugar and 200-300 grams of sodium per serving.
Where is the FDA in the Process?
The FDA published the proposed rule for Healthy on September 29, 2022, and the comment period was extended to February 16, 2023. All comments have been gathered and are being reviewed by the FDA. Though there is not a clear deadline, our guess is that the final rule will be published in the Q3 2023, with a mandatory implementation date sometime in 2025.

FDA: Proposed Healthy Labels to Be Utilized with the Proposed Healthy Rule
Additionally, last month, the FDA released industry draft guidance for the voluntary front-of-package labeling including packaging, graphics, and websites. The agency explained the guidance is meant to, “support the use of more nutrition-related labeling statements that focus on foods and food groups in relation to nutritious eating patterns.” They hope this change will encourage reformulation and promote healthy food and beverage innovation for more better-for-you products on the market. The front-of-package symbols developed for packaging are currently going through consumer testing. The deadline for comment submission on the FDA’s draft guidance is June 26, 2023. Electronic comments will be made public. Further information is available at the Federal Regulations website
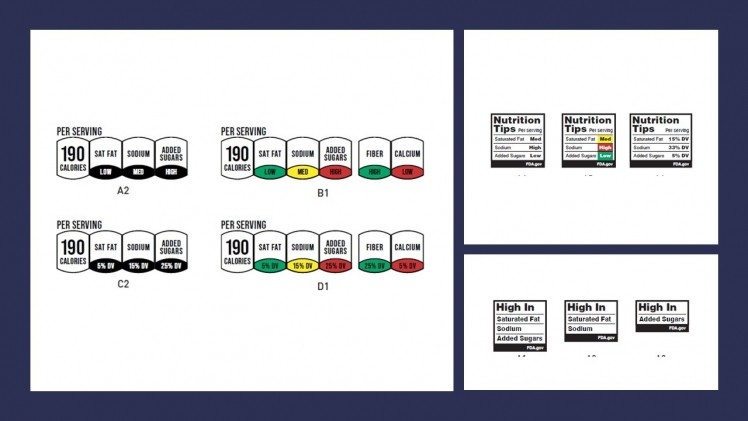
FDA: Front-of-Package Proposed Rule Symbols Currently in Review
The United States is not the leader in labeling packaged food with a health-forward approach, but with the desire across cultures to actively manage one’s health it seems they’re starting to take a page out of books from other nations. Israel has a few labels already adorning many of their packages, including one which translates to “sugar in high quantities”. Australia and New Zealand already rely on a Health Star rating, which uses a scale from .5 to 5 stars, the latter signifying a healthier item. The European Union has two classifications for their labels, nutrient-specific and summary indicator. The former provides the percentage of nutrients compared to the daily recommendation. The latter includes healthiness labels like the Nutri-Score symbol that indicates the healthiness of the product by a graded system comprised of five letter and color combinations. It is only a matter of time before consumers become a louder voice in the conversation and demand more transparency and balance throughout the CPG industry.
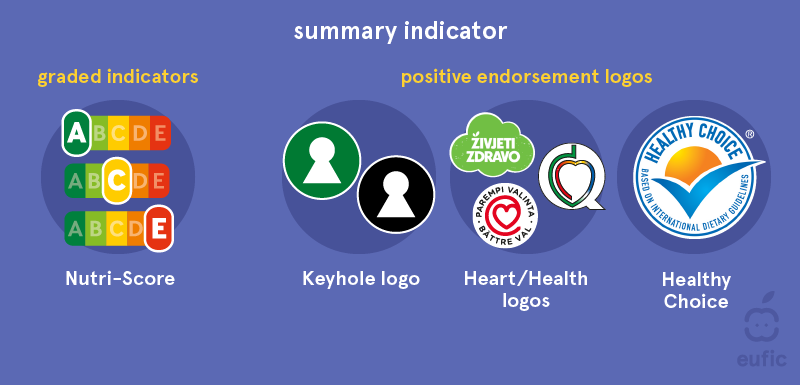
European Food Safety Authority: Front-of-Packaging Labeling Schemes
We don’t know what the outcome of this conversation will be, but we do know that the health and wellness trend isn’t going away. If you need to reformulate or wish to develop something completely new, we provide the expertise required. Our flavorists, product developers, taste modulation and sensory scientists, regulatory gurus, engineering experts and others will collaborate to ensure that our ingredients, formulations, and scale-up support deliver your intended product impact.
Stay tuned for Part 2 — a conversation on food and beverage innovation, high impact sweeteners, consumer demand, and the effectiveness of package labeling.
Contact us through our website or email marketing@imbibeinc.com to continue the conversation and see how Imbibe’s R&D and Regulatory departments can fortify your portfolio.